Gametia European Biobank
Gametia Biobank is an international gamete bank that covers an essential activity in the world of human reproduction: reliably providing clinics, and the professionals who work in them, of the top quality gametes to safely perform fertility treatments to all those patients who need a donor.
"We all know that practice and precision lead every laboratory to excellence, there are no shortcuts

since 2020
a year

from our gametes
Gametia is a new brand, but we have been dedicated to biobanking for decades, mainly in Spain and Italy, extending a single standard of care in all our centres. Currently we count with 7 centres in 3 countries which allows to provide a large phenotype availability.
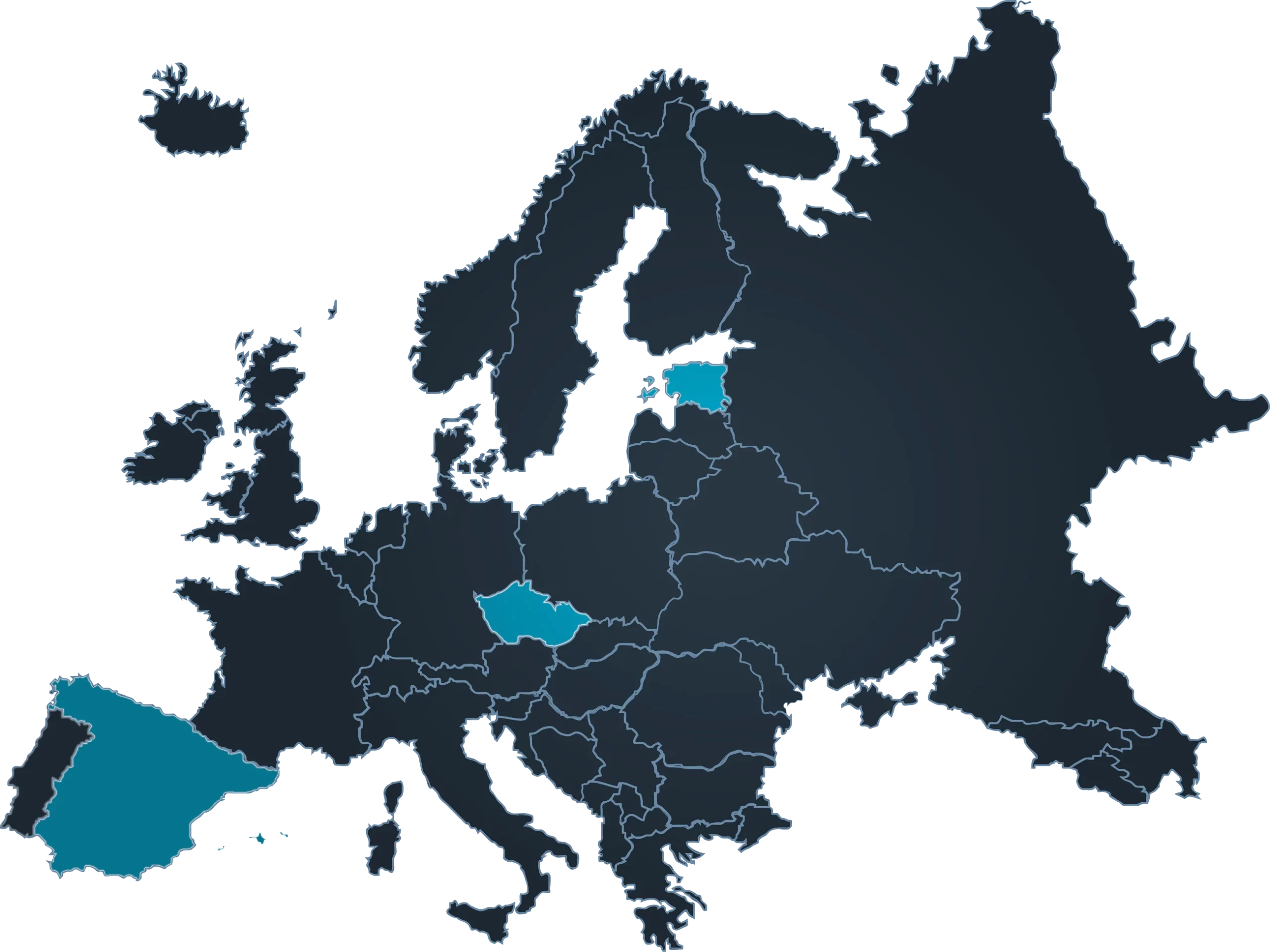
"Gametia Biobank does not take risks, so that you do not run them either".
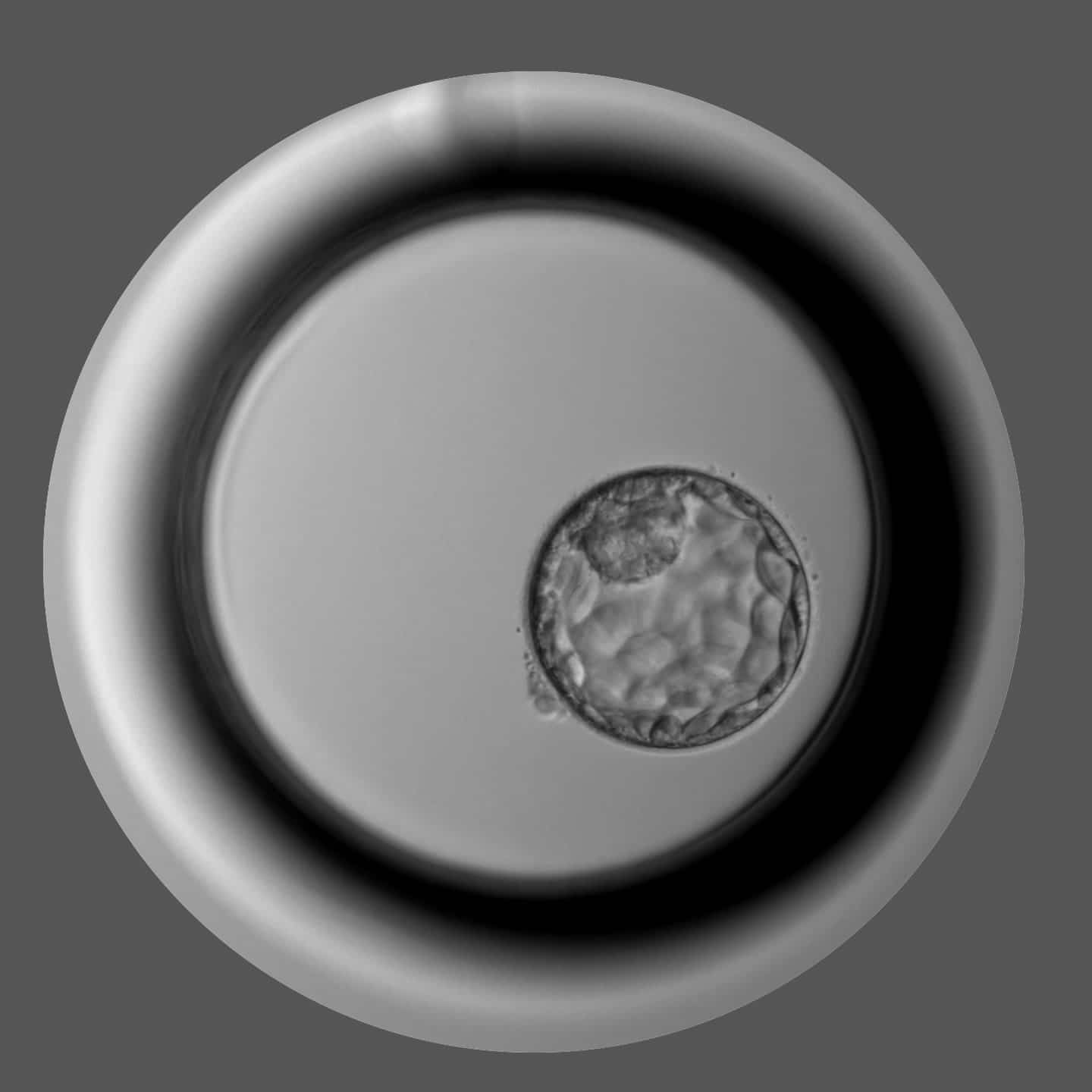
Gametia aims to become the first European Biobank with the largest open and anonymous donor base with wide phenotype variability, highest quality standards, and service reputation that is able to supply donor biomaterial to the leaders in the IVF world.