Gametia European Biobank
Choose the Quality, Transparency and Safety of Europe’s leading Biobank.


Gametia Biobank is an international gamete bank that covers an essential activity in the world of human reproduction: reliably providing clinics, and the professionals who work in them, of the top quality gametes to safely perform fertility treatments to all those patients who need a donor.
"We all know that practice and precision lead every laboratory to excellence, there are no shortcuts

in the last 5 years

a year

from our gametes
We have been dedicated to biobanking for decades. Currently we count with 8 centres in 2 countries, all of them working under the same high-quality standards of care. We can provide a large phenotype availability for both anonymous (Spain) and open ID donors (Portugal).

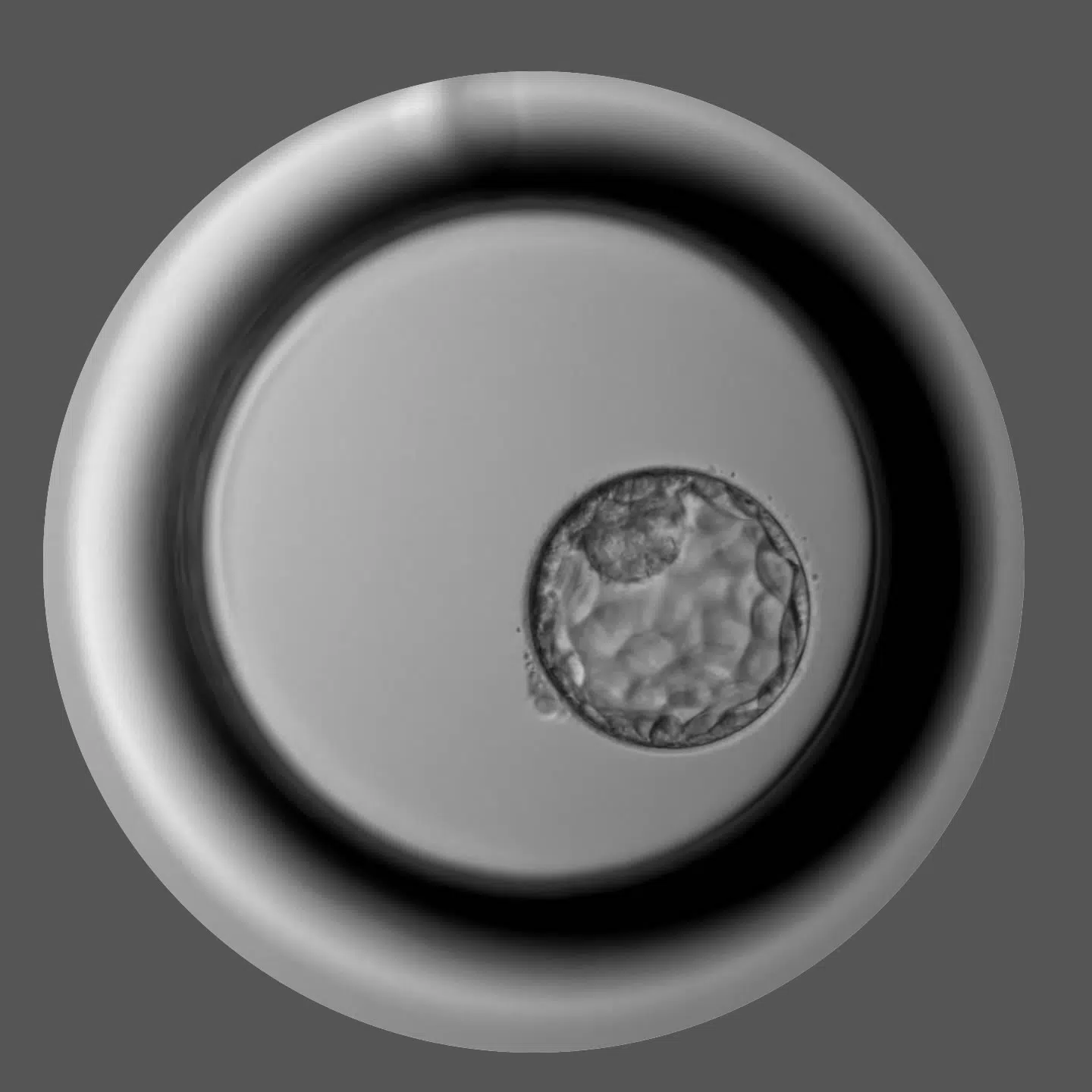
We put at your service the good work of our team, our technology and equipment, our clinical processes, our accompaniment and commitment to your results, our total transparency and compliance with current legislation, and, in short, the quality that comes from our way of working and the security that comes from it.
And what do we mean when we talk about quality?
- Security in donor selection and assignment processes.
- Safety, thanks to exhaustive genetic testing.
- Safety in the laboratory – traceability, identity control, high standards of work and use of leading marks.
- Shipment security – traceability and reception conditions, deadlines.
- Certainty in results – survival rates, blast arrival rates.
"Gametia Biobank does not take risks, so that you do not run them either".

Gametia aims to become the first European Biobank with the largest open and anonymous donor base with wide phenotype variability, highest quality standards, and service reputation that is able to supply donor biomaterial to the leaders in the IVF world.